HiSET Science Study Guide: Physical Science
Physical science questions will assess your knowledge and understanding of:
- Physical properties
- The relationship between motion and position
- Light, heat, electricity, and magnetism
- Matter and atomic structures
- Chemical reactions
Chemistry
Chemistry is a vast branch of physical science that studies the matter that makes up all things. About 100 elements exist in nature, the basic building blocks of all things. We start our journey with the smallest part of any element: the atom.
Atoms
All atoms have three basic parts:
- Neutrons: Neutral particles that make up part of the atom’s nucleus.
- Protons: Positively charged particles that make up part of the atom’s nucleus.
- Electrons: Negatively charged particles that orbit the nucleus, much like how planets orbit the Sun.
An element’s atomic number refers to how many protons exist in the nucleus. A higher number also indicates a higher atomic mass. However, even the heaviest atoms are minuscule compared to the period at the end of this sentence.
Atoms can bond with other atoms in two ways. In a covalent bond, two atoms share electrons. In an ionic bond, one atom gives up one or more electrons to another atom. When two atoms of the same or different elements create a bond, they form a molecule. Some complex molecules, such as DNA, contain thousands of atoms.
Molecules can react with one another in chemical reactions. Chemists use chemical formulas to express these reactions, such as:
4Fe + 3O2 + 6H2O → 4Fe(OH)3
In this chemical reaction, three reactants (iron, oxygen, and water) react with one another. The arrow points to the product, rust.
Although atoms can share or give up their electrons, it’s nearly impossible to break apart their nucleus. However, the discovery of nuclear fission (e.g. atomic power plants and nuclear bombs) changed the course of human history.
Chemistry and Life
Organic chemistry is the branch of chemistry that studies carbon compounds, such as those responsible for all life on Earth: DNA. Our DNA is an example of a polymer, a very large molecule. Organic chemists also study hydrocarbons, long chains of hydrogen and carbon molecules, such as those that make up gasoline and other fuel.
Matter
Matter has mass and weight. Mass is the quantity of matter in an object, while weight describes the force of gravity on that object. For example, 1 kilogram of gold will weigh less on the Moon because there is less gravity, even though the mass remains the same.
There’s more to know about matter than solids, liquids, and gases. For example, watching an ice cube melt or freeze is a physical change, or a change in which no chemical reaction takes place. However, when you turn granulated sugar into dark caramel, you’re witnessing an important chemical change wherein your pan’s heat is causing the sugar molecules to turn into something new and tasty.
Staying in the kitchen, imagine you have a sore throat and decide to gargle salt water. The water in your glass is a solvent, a substance that can dissolve other substances. The salt you add to the glass is the solute, the thing being dissolved. What you create by mixing them together is a solution. However, if you add too much salt, you’ll saturate your solution, meaning the water cannot dissolve any more salt.
Chemical Changes
Chemical reactions fall into two categories: decomposition reactions and combination reactions. In a decomposition reaction, a complex substance breaks down into simpler substances. In a combination reaction, simpler substances come together to form a more complex product. In both cases, the original substances are called reactants, and the result is the product.
No matter how dramatic a chemical reaction may appear, it doesn’t create or destroy matter. According to the law of conservation of matter, the total amount of matter stays the same during a reaction—atoms are simply rearranged. The only exception involves nuclear reactions like fission, in which atoms themselves are split apart. These reactions are not chemical but nuclear, and they release enormous amounts of energy.
Physics
Physics is the study of the interaction among matter, energy, and motion. Since we’ve already covered matter, we’ll spend our time in this section discussing energy and motion, as well as a few other physics topics you should know.
Energy
Energy is a BIG topic, but you can prepare for the HiSET science test by reviewing these 10 need-to-know vocabulary words.
Calorie: A unit of energy you often find on food packaging. One calorie is the energy needed to raise the temperature of 1 gram of water by 1 degree Celsius.
Electricity: The flow of electrons in a system. This process causes an electrical charge.
Entropy: The amount of disorder in a system. Over time, systems naturally become more disordered; this is called increasing entropy. For example, when wood burns in a campfire, it turns into ash, smoke, and gas. The matter and energy become more spread out, increasing the system’s disorder.
Infrared Rays: Light with a long wavelength that cannot be seen by the naked eye. However, special machines can view these rays and represent them in a way we can see on a computer screen.
Kinetic Energy: The energy something has when it is in motion. If a car crashes into something that is still, it transfers its kinetic energy to the other object, causing damage.
Magnetic Energy: The energy within a magnetic field. You can feel this energy if you push together or pull apart two magnets.
Potential Energy: As the name suggests, potential energy is the amount of energy something possesses in relation to other objects. A ball at the top of a hill has a lot more potential energy than a ball at the bottom because it can roll down the hill.
Solar Energy: The energy stars like our Sun emit. It can be visible (e.g. the light we see) or invisible (e.g. UV radiation) energy. Plants use solar energy to perform photosynthesis.
Radiation: Energy that moves between two places. Depending on the wavelength, it can appear as visible light, UV radiation, or X-rays. The shorter the wavelength, the more radioactive and potentially dangerous a type of radiation is.
Wavelength: The distance between two crests of a wave of light. When used to represent radiation, a shorter wavelength indicates higher energy. High-wavelength forms of radiation include radio and television waves.
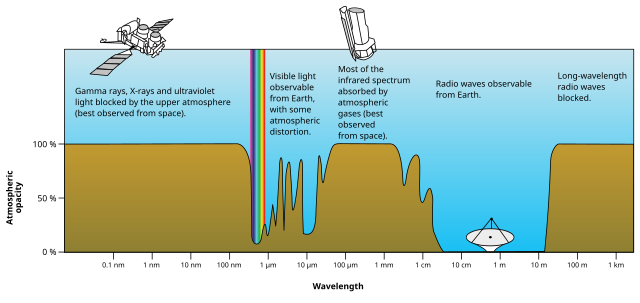
Source: NASA (original); SVG by Mysid., Public domain, via Wikimedia Commons
Visible light is a type of radiation that exists in a narrow wavelength. Most radiation doesn’t even reach the Earth’s surface due to the atmosphere.
Motion
To understand motion, you need to know the similarities and differences between these key terms.
Acceleration: An object’s change in velocity. It’s why, if you push down on a car’s accelerator, the car will go faster.
Momentum: The relationship between an object’s mass and velocity. An object with little mass can have a high momentum if it’s going fast, and an object with a large mass can have a high momentum if it’s going slow.
Speed: The rate at which an object covers a specific distance (e.g. a car going 60 miles per hour).
Velocity: Similar to speed, but velocity also considers the direction an object is moving.
Force and Work
Force is when you push on your shopping cart or pull on a rope. Work refers to how much energy is transferred when you apply force. For example, pushing an empty shopping cart 10 feet takes less force than pushing a full shopping cart 10 feet. In both cases, you perform the same amount of work.